 资源预览
资源预览
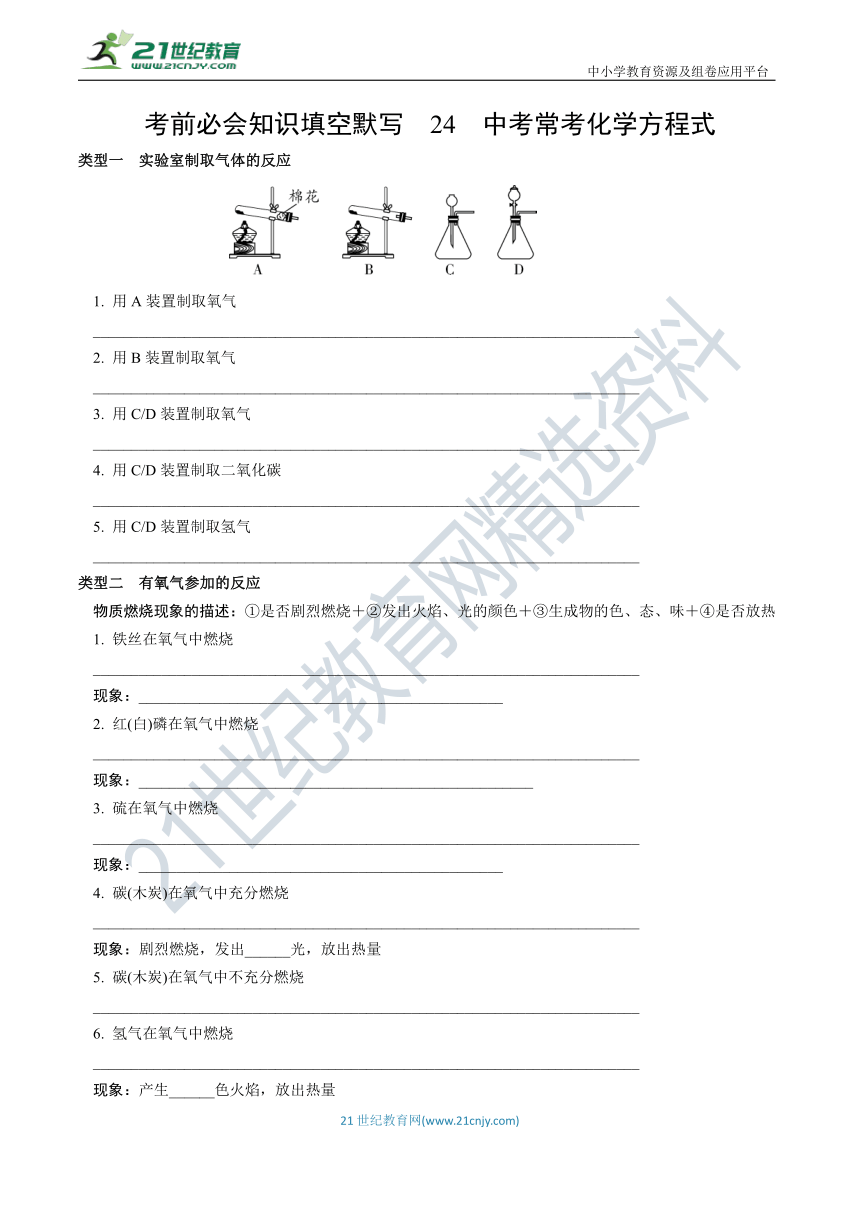

 资源预览
资源预览




